Newtonian Mechanics don't work in the quantum world.
Quantum objects are very microscopic that they can't be located but they can be probably located. And this probability can be determined by using wave functions of the quantum particles

Wave function gives us the probabilities of where the electron is likely to be. The act of not knowing where the electron is, allows its probability distribution to be spread out over a large space kind of wave. Therefore wave function is the function which describes the wave shape of the probability distribution of the electron.
The Heisenberg Uncertainty principle says that we can't predict the exact position and momentum of the quantum objects. But we can know about things like Energy levels and wave functions.
THE SCHRODINGERS WAVE EQUATION :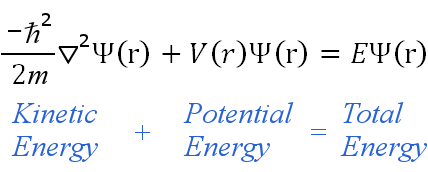

And we know the famous Einsteins equation E=hf where h is Planks constant. Since only a few frequencies are allowed only certain energy levels are allowed.
That is what is meant by QUANTISATION.
Derivation :
For more detailed information :
http://physics.mq.edu.au/~jcresser/Phys201/LectureNotes/SchrodingerEqn.pdf
http://lejpt.academicdirect.org/A26/031_048.pdf
Quantum objects are very microscopic that they can't be located but they can be probably located. And this probability can be determined by using wave functions of the quantum particles

Wave function gives us the probabilities of where the electron is likely to be. The act of not knowing where the electron is, allows its probability distribution to be spread out over a large space kind of wave. Therefore wave function is the function which describes the wave shape of the probability distribution of the electron.
The Heisenberg Uncertainty principle says that we can't predict the exact position and momentum of the quantum objects. But we can know about things like Energy levels and wave functions.
THE SCHRODINGERS WAVE EQUATION :
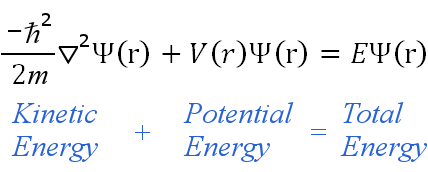
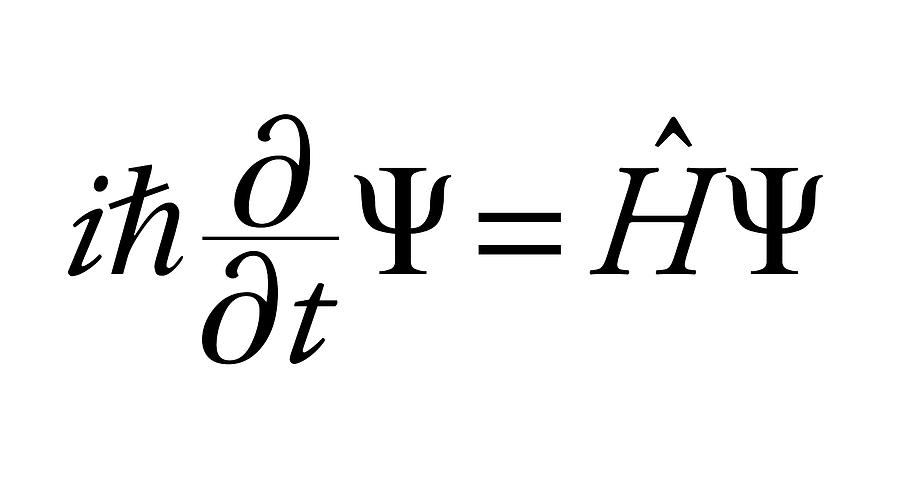
 = Wave function (psi) E= Energies of the electron is allowed to have
= Wave function (psi) E= Energies of the electron is allowed to have
E[(psi)(x)] = The energy levels of the wave function of the electron
If we consider an electron inside a box where it is confined to only a few frequencies the wavefunction always needs to be 0. In other words, the electron has 0 probability to be found outside the box.

And we know the famous Einsteins equation E=hf where h is Planks constant. Since only a few frequencies are allowed only certain energy levels are allowed.
That is what is meant by QUANTISATION.
Derivation :
For more detailed information :
http://physics.mq.edu.au/~jcresser/Phys201/LectureNotes/SchrodingerEqn.pdf
http://lejpt.academicdirect.org/A26/031_048.pdf



