LASERs play a very vital role in many fields and industries.
What makes it so special?
It's characteristics.
Lasers have:
1.High directionality
2.High monochromaticity
3.A high degree of coherence
4.High brightness


So let's understand each one in detail.
1. High directionality:
The lasers are made of active medium placed in between two reflecting resonator mirrors one which can partially reflect and the other which can fully reflect the light.
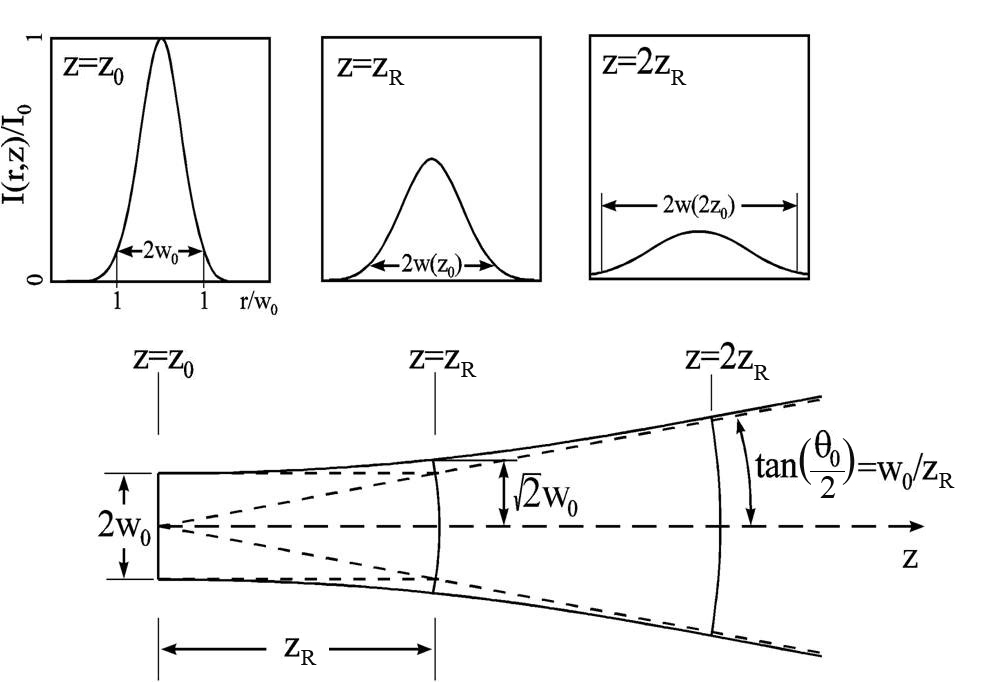
Any photon travelling in a direction away from the cavity axis reflects away by the mirrors within a few reflections and is thus not allowed to propagate further, thus the beam drawn from the output mirror is highly parallel and directional. The degree of directionality is expressed as the divergence.
Minimum spot point: The curvature of the mirror confines the light within the cavity and causes the beam to narrow down to a radius wo and this is called a minimum spot point.
Any photon travelling in a direction away from the cavity axis reflects away by the mirrors within a few reflections and is thus not allowed to propagate further, thus the beam drawn from the output mirror is highly parallel and directional. The degree of directionality is expressed as the divergence.
Minimum spot point: The curvature of the mirror confines the light within the cavity and causes the beam to narrow down to a radius wo and this is called a minimum spot point.
The beam of divergence phi is equal to 1.22*wavelength / 2*wo
The divergence tells us how rapidly the beam spreads when its emitted.
For a laser, the beam divergence is 1milliradian.
The angle of divergence = (a2-a1) / 2*(d2-d1)
where d1 and d2 distances from the laser window and a1 and a2 are the diameters of the spot.
HIGH MONOCHROMATICITY:
The light emitted from a laser is monochromatic, that is, it is of one wavelength (colour).
The emission or absorption of the photons whose frequency lies between f and f+df which is denoted as spectral broadening.
The three important mechanisms which rise to the spectral broadening are :
1. Doppler broadening: The atoms emitting and absorbing photons are not at rest. They move with some velocity in a probable region within the atom. Thus the frequency of emitted radiation changes slightly and this is called doppler broadening.
2.Collision broadening: During the emission or absorption if the atoms get collided then resulting frequency gets effected and this is known as collision broadening.
3.Natural broadening: In solid materials emitting photons leads to damping of the amplitude of the wave train and this is known as natural broadening.
Spectral width or line width is a quantity which is used to find the degree of monochromaticity of light.
change in wavelength = - (c* change in frequency) / ((frequency)^2)
For lasers change in wavelength is approx. 0.001nm and can be made much narrower.