SCHRODINGER'S CAT is a thought experiment by Erwin Schrodinger to make the world understand that quantum particles exist in a superposition.
Quantum theory states that quantum particles can achieve more than one state simultaneously.
SCHRODINGER'S CAT EXPERIMENT:
If we put a cat in a strong box with a radioactive sample which has 50 per cent of chances of decaying and closes the box, it is very confusing for the observer outside to guess whether the cat is dead or alive after a period of time. 
So before opening the box, we can say that the cat is in superposition. That is it's in the state of both dead and alive. But if we open the box and observe we may find the cat in only one state that is the state of dead or alive.

This means that quantum particles exist in different states but when observed takes a single state.
And according to multiverse theory or many worlds theory, the universe achieves all the possible outcomes by splitting itself and making its copies. So from the thought experiment, we can say that the cat is alive and dead in the split universe.
That means the quantum particles achieve all the possible positions one in each copy of the universe.
For further understanding :
https://www.aps.org/units/maspg/meetings/upload/franson-021815.pdf
https://www3.nd.edu/~jspeaks/courses/2007-8/20229/_HANDOUTS/quantum-mechanics.pdf

This means that quantum particles exist in different states but when observed takes a single state.
And according to multiverse theory or many worlds theory, the universe achieves all the possible outcomes by splitting itself and making its copies. So from the thought experiment, we can say that the cat is alive and dead in the split universe.
That means the quantum particles achieve all the possible positions one in each copy of the universe.
For further understanding :
https://www.aps.org/units/maspg/meetings/upload/franson-021815.pdf
https://www3.nd.edu/~jspeaks/courses/2007-8/20229/_HANDOUTS/quantum-mechanics.pdf
Physics tells us observations can't be predicted absolutely. Rather, there's a range of possible observations each with a different probability.


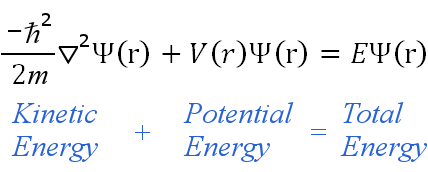
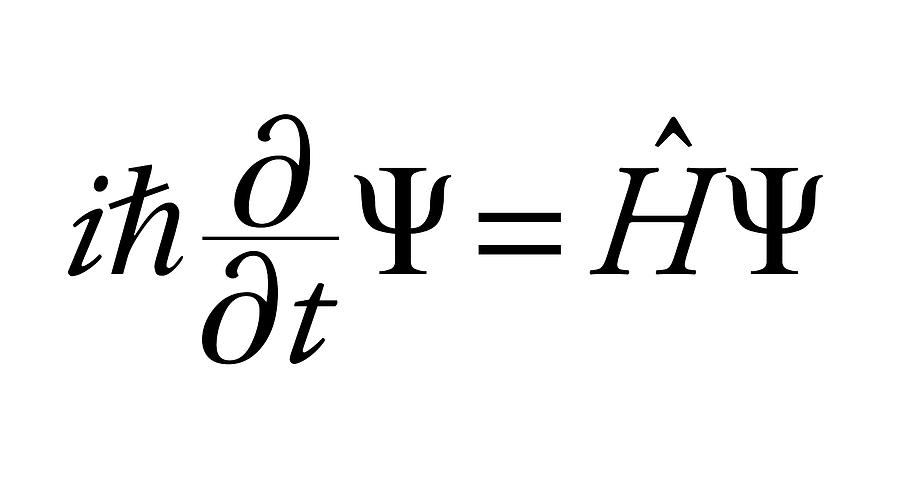
 = Wave function (psi) E= Energies of the electron is allowed to have
= Wave function (psi) E= Energies of the electron is allowed to have 
